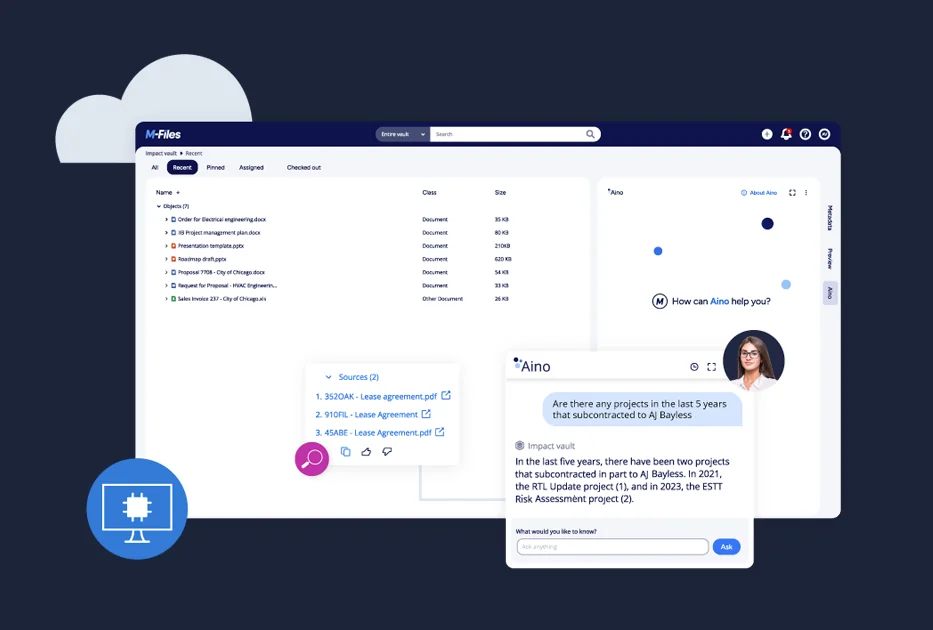
Quality Management System
Improve quality control by embedding your quality management system into daily business processes. Leverage controlled document management and learning requirements to ensure your staff is always using up-to-date procedures. Automate traceability of issues, corrective and preventive actions. Streamline audits with automated evidence from version control and audit trails.

The Future of Quality Management
Learn how M-Files can help your organization boost employee productivity, ensure accessible information, and align workflows with company policies.

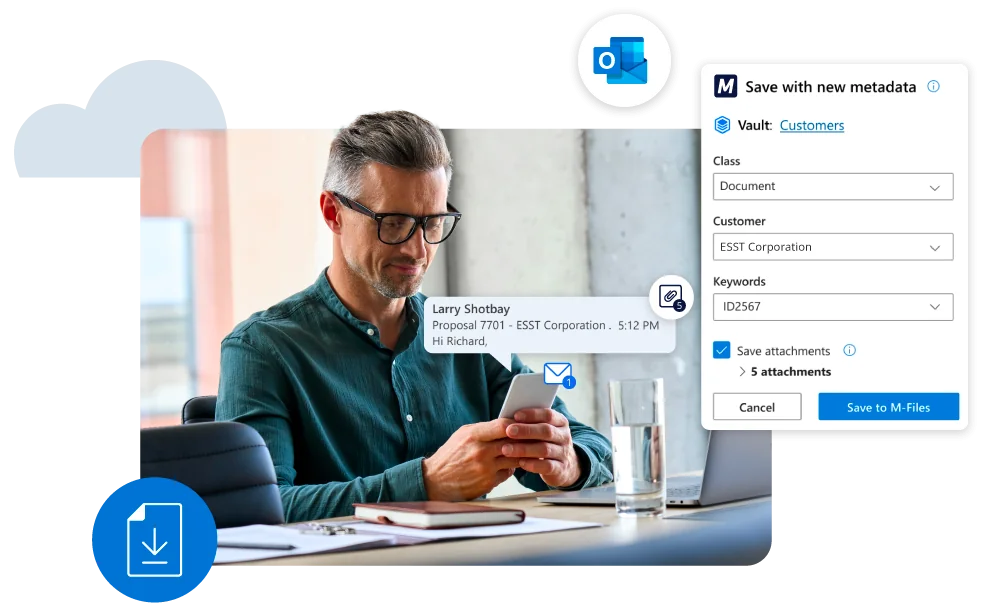
Challenges: Quality management relies on manual work and employee diligence
Any organization benefits from documenting processes and establishing ways to monitor and improve outcomes. That is the essence of quality management, but many organizations struggle with implementing a QMS in their daily business without dedicated staff and systems. Sometimes, despite all the efforts, the impact of the activity is reduced to getting a certificate without desirable business benefits.
Quality Management
Boost Operational Performance through Quality Management
To succeed, organizations need a quality management strategy that enhances employee productivity, streamlines access to information, and aligns workflows with organizational standards. Download the brochure to discover how to enhance operational efficiency for your organization.

Solutions: An electronic quality management system that is integrated to daily work
Embedding quality management processes into daily work drives adoption and automates manual tasks. Automated version control and audit trails form a basis for a traceable way of working. Integrating standard operating procedures into workflows, templates, and assignments help implement new processes without extra effort. Audits can also be completed without extra preparations as all evidence is already built into the daily work.
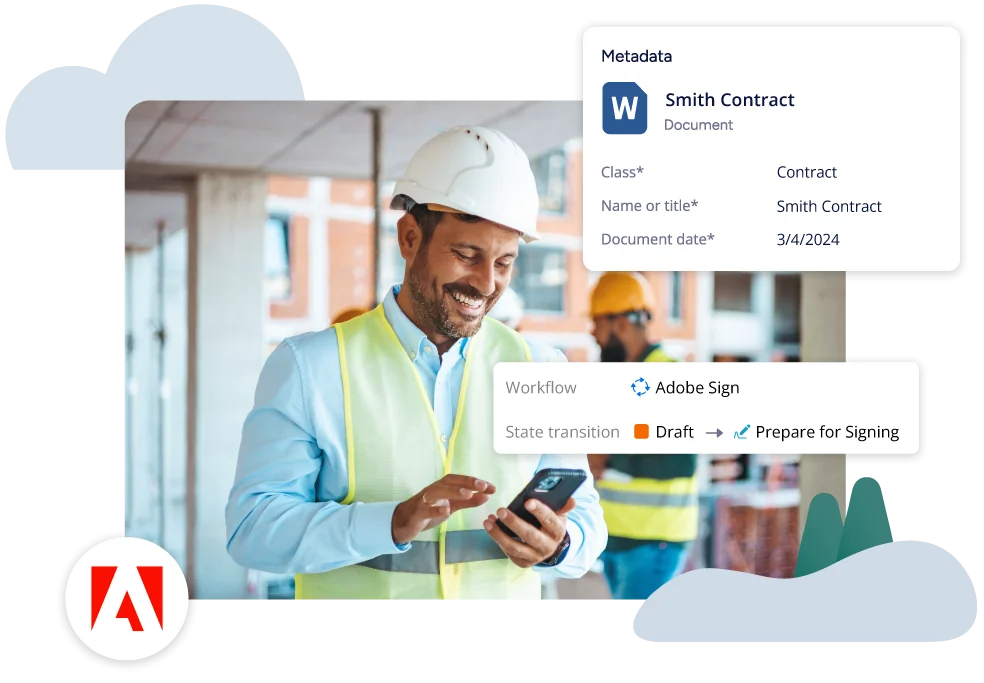
Solutions for Your Industry
EQMS helps manufacturing improve operational efficiency
An integrated eQMS solution streamlines and automates quality processes, ensuring compliance with industry standards and regulatory requirements. It enhances visibility and control over quality data, facilitating real-time monitoring and swift corrective actions. Additionally, eQMS software promotes continuous improvement by enabling data-driven decision-making and collaboration across departments, ultimately leading to higher product quality and operational efficiency.
Integrated quality management drives compliance in Insurance
An integrated eQMS (Electronic Quality Management System) enhances efficiency and compliance for insurance brokers by automating and streamlining document management, client interactions, and regulatory reporting. It improves data accuracy and consistency, enabling brokers to deliver better service to their clients and market, while maintaining compliance with industry regulatory standards. Additionally, eQMS promotes transparency and accountability, facilitating audits and continuous process improvement.
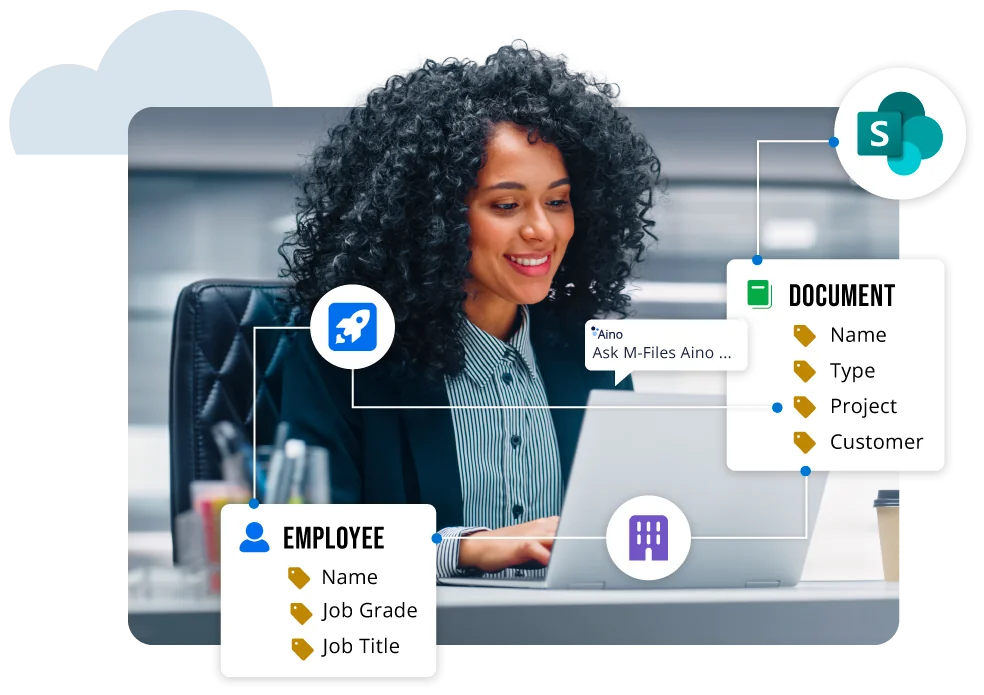
See M-Files in Action Now
Watch our interactive product tour. No wait. No scheduling. Start exploring our AI-powered document management system with a demo tailored to your use case.

Customer Case Studies
More than 5,000 companies rely on M-Files to manage their documents every day.